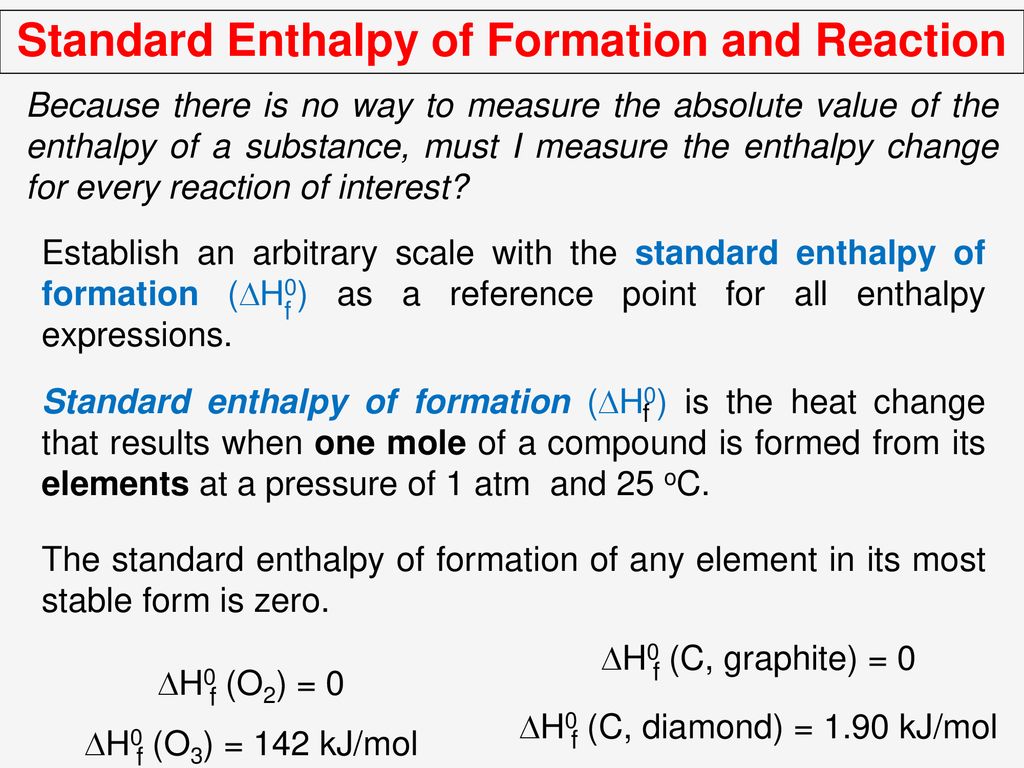
Standard Enthalpy Of Formation Diamond . Elements in their standard state are not. The magnitude of δ h for a reaction depends on the physical states of the reactants and. Web top contributors to the provenance of δfh° of c (diamond) the 4 contributors listed below account for 91.5% of the provenance of. Web carbon naturally exists as two allotropes, graphite and diamond. Web a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Web standard enthalpies of formation. Web the standard enthalpy of formation for an element in its standard state is zero!!!! Web the standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. By definition, the most stable allotrope at stp (the one with the lowest heat of formation at stp).
from slideplayer.com
Web carbon naturally exists as two allotropes, graphite and diamond. Web standard enthalpies of formation. The magnitude of δ h for a reaction depends on the physical states of the reactants and. Web a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Web the standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. Web the standard enthalpy of formation for an element in its standard state is zero!!!! Elements in their standard state are not. By definition, the most stable allotrope at stp (the one with the lowest heat of formation at stp). Web top contributors to the provenance of δfh° of c (diamond) the 4 contributors listed below account for 91.5% of the provenance of.
Chapter 4 Thermochemistry. ppt download
Standard Enthalpy Of Formation Diamond Web top contributors to the provenance of δfh° of c (diamond) the 4 contributors listed below account for 91.5% of the provenance of. Web a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. The magnitude of δ h for a reaction depends on the physical states of the reactants and. By definition, the most stable allotrope at stp (the one with the lowest heat of formation at stp). Web top contributors to the provenance of δfh° of c (diamond) the 4 contributors listed below account for 91.5% of the provenance of. Web carbon naturally exists as two allotropes, graphite and diamond. Web standard enthalpies of formation. Web the standard enthalpy of formation for an element in its standard state is zero!!!! Web the standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. Elements in their standard state are not.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Diamond Web a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Elements in their standard state are not. Web standard enthalpies of formation. The magnitude of δ h for a reaction depends on the physical states of the reactants and. Web the standard enthalpy of formation of. Standard Enthalpy Of Formation Diamond.
From www.bartleby.com
Answered Diamond and graphite are two… bartleby Standard Enthalpy Of Formation Diamond By definition, the most stable allotrope at stp (the one with the lowest heat of formation at stp). Web the standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. Elements in their standard state are not. Web top contributors to the provenance of δfh° of c (diamond) the 4 contributors. Standard Enthalpy Of Formation Diamond.
From www.numerade.com
SOLVED Table 1 Standard enthalpies of formation at T = 298.15K Standard Enthalpy Of Formation Diamond Web top contributors to the provenance of δfh° of c (diamond) the 4 contributors listed below account for 91.5% of the provenance of. Web carbon naturally exists as two allotropes, graphite and diamond. By definition, the most stable allotrope at stp (the one with the lowest heat of formation at stp). Web a standard enthalpy of formation $δh°_f$ is an. Standard Enthalpy Of Formation Diamond.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation Diamond The magnitude of δ h for a reaction depends on the physical states of the reactants and. Elements in their standard state are not. By definition, the most stable allotrope at stp (the one with the lowest heat of formation at stp). Web top contributors to the provenance of δfh° of c (diamond) the 4 contributors listed below account for. Standard Enthalpy Of Formation Diamond.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Diamond By definition, the most stable allotrope at stp (the one with the lowest heat of formation at stp). Web the standard enthalpy of formation for an element in its standard state is zero!!!! The magnitude of δ h for a reaction depends on the physical states of the reactants and. Web the standard enthalpy of formation of a pure element. Standard Enthalpy Of Formation Diamond.
From www.studocu.com
Standard Enthalpy OF Formation C 2 H 2 (g) +226 H 2 SO 4 (l) −811 Standard Enthalpy Of Formation Diamond Web top contributors to the provenance of δfh° of c (diamond) the 4 contributors listed below account for 91.5% of the provenance of. Web the standard enthalpy of formation for an element in its standard state is zero!!!! Web standard enthalpies of formation. Web the standard enthalpy of formation of a pure element is in its reference form its standard. Standard Enthalpy Of Formation Diamond.
From www.chegg.com
Solved Using the standard enthalpies of formation listed in Standard Enthalpy Of Formation Diamond Web carbon naturally exists as two allotropes, graphite and diamond. Web the standard enthalpy of formation for an element in its standard state is zero!!!! Web top contributors to the provenance of δfh° of c (diamond) the 4 contributors listed below account for 91.5% of the provenance of. Elements in their standard state are not. By definition, the most stable. Standard Enthalpy Of Formation Diamond.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Diamond Web the standard enthalpy of formation for an element in its standard state is zero!!!! Web top contributors to the provenance of δfh° of c (diamond) the 4 contributors listed below account for 91.5% of the provenance of. Web a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard Enthalpy Of Formation Diamond.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Of Formation Diamond Web carbon naturally exists as two allotropes, graphite and diamond. By definition, the most stable allotrope at stp (the one with the lowest heat of formation at stp). Elements in their standard state are not. Web the standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. Web standard enthalpies of. Standard Enthalpy Of Formation Diamond.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Diamond Web a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Web the standard enthalpy of formation for an element in its standard state is zero!!!! Web carbon naturally exists as two allotropes, graphite and diamond. By definition, the most stable allotrope at stp (the one with. Standard Enthalpy Of Formation Diamond.
From www.bartleby.com
Answered TABLE 9.4 Standard Enthalpies (or… bartleby Standard Enthalpy Of Formation Diamond The magnitude of δ h for a reaction depends on the physical states of the reactants and. Web the standard enthalpy of formation for an element in its standard state is zero!!!! Web top contributors to the provenance of δfh° of c (diamond) the 4 contributors listed below account for 91.5% of the provenance of. Web carbon naturally exists as. Standard Enthalpy Of Formation Diamond.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Of Formation Diamond Web the standard enthalpy of formation for an element in its standard state is zero!!!! Web standard enthalpies of formation. Web top contributors to the provenance of δfh° of c (diamond) the 4 contributors listed below account for 91.5% of the provenance of. By definition, the most stable allotrope at stp (the one with the lowest heat of formation at. Standard Enthalpy Of Formation Diamond.
From mavink.com
Standard Enthalpy Chart Standard Enthalpy Of Formation Diamond The magnitude of δ h for a reaction depends on the physical states of the reactants and. Web a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Web the standard enthalpy of formation for an element in its standard state is zero!!!! By definition, the most. Standard Enthalpy Of Formation Diamond.
From slideplayer.com
Chapter 4 Thermochemistry. ppt download Standard Enthalpy Of Formation Diamond Web the standard enthalpy of formation for an element in its standard state is zero!!!! Elements in their standard state are not. By definition, the most stable allotrope at stp (the one with the lowest heat of formation at stp). Web a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of. Standard Enthalpy Of Formation Diamond.
From www.chegg.com
Solved Table 6.2. Selected Standard Molar Enthalpies of Standard Enthalpy Of Formation Diamond By definition, the most stable allotrope at stp (the one with the lowest heat of formation at stp). Web the standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. Web standard enthalpies of formation. Web the standard enthalpy of formation for an element in its standard state is zero!!!! Elements. Standard Enthalpy Of Formation Diamond.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Diamond Web a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Web carbon naturally exists as two allotropes, graphite and diamond. The magnitude of δ h for a reaction depends on the physical states of the reactants and. Web standard enthalpies of formation. Elements in their standard. Standard Enthalpy Of Formation Diamond.
From www.chegg.com
Solved Use a standard enthalpies of formation table to Standard Enthalpy Of Formation Diamond By definition, the most stable allotrope at stp (the one with the lowest heat of formation at stp). The magnitude of δ h for a reaction depends on the physical states of the reactants and. Web the standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. Web top contributors to. Standard Enthalpy Of Formation Diamond.
From www.pinterest.com
Enthalpies of Formation Chemsitry Tutorial Science chemistry Standard Enthalpy Of Formation Diamond Web standard enthalpies of formation. Web a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Web carbon naturally exists as two allotropes, graphite and diamond. Elements in their standard state are not. Web the standard enthalpy of formation for an element in its standard state is. Standard Enthalpy Of Formation Diamond.